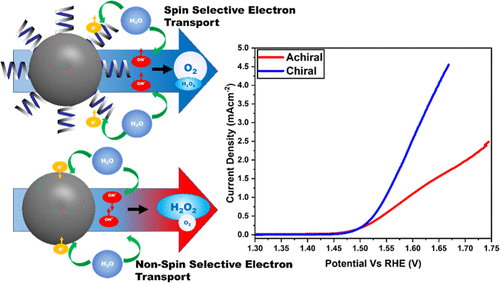
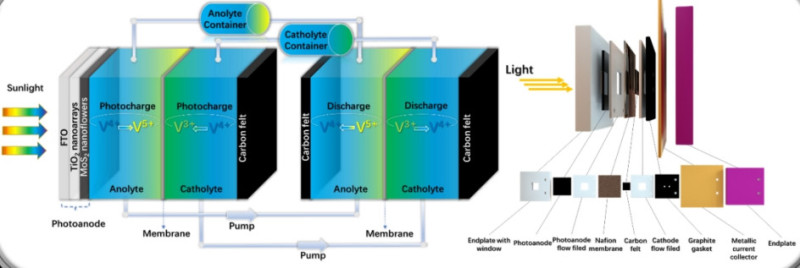
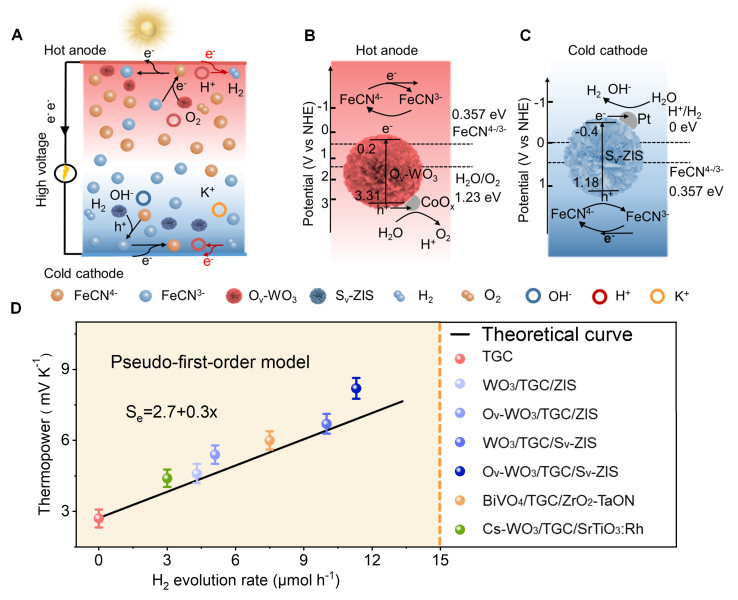
Maria and Ana presented their approaches to sustainable energy storage at the Night of Science and Engineering, held at The Octagon, on the 29th February 2024.
We need batteries to store energy from renewables to be able to cope with their inherent intermittency and move away from fossil fuels.
However, those batteries should also be engineered with sustainable and abundant resources that do not rely on fossil fuels either.
At the Night of Science and Engineering, they showcased their approaches to sustainable batteries based on the use of recycled materials and biomass resources combined with innovative processing techniques.
Thanks Carlos, Hattie and Michael! It was such a great event. Feel very proud of you all! 🙂