- For a complete list of publications, please follow this link
Y. Wen, T. P. Neville, A. Jorge Sobrido, P. R. Shearing, D. J. L Brett, R. Jervis, J Power Sources 2023, 566, 232861.
The current obstacles for all-vanadium redox flow batteries (VRFBs) include the sluggish reaction kinetics of electrode materials and the overlapping potential range of the hydrogen evolution reaction (HER) with the negative redox couple. Bismuth (Bi) additives have exhibited tremendous enhancement of battery performance; however, the performance plateaus with concentration of Bi and the catalytic mechanism remains inconclusive and controversial. Quantified kinetic values from three electrochemical methodologies, including modelling, show that the performance plateau with Bi concentration was related to the kinetics of the vanadium redox reaction (VRR) instead of dissolution of Bi, and VRR reaction rate was improved three orders of magnitude with the addition of Bi, with the highest VRR reaction rate observed for electrolyte with 750 ppm Bi additive. Additionally, a competing relationship between VRR and the HER was explored via electrochemical methodologies. It was further confirmed that Bi effectively selectively catalysed VRR over HER via in-situ mass spectrometry measurements during battery cycling, which allows for a higher battery charging cut-off potential of 1.7 V without electrolyte imbalance caused by water electrolysis, achieving 86% of the theoretical electrolyte capacity at 110 mA cm−2 with 750 ppm Bi. The full battery performance with 750 ppm Bi additive is among the best in the literature to date.

Y. Zhang, Y. Li, X. Xin, Y. Wang, P. Guo, R. Wang, B. Wang, W. Huang, A. Jorge Sobrido, X. Li, Nature Energy 2023, https://doi.org/10.1038/s41560-023-01242-7.
Multiple exciton generation (MEG), where two or more electron–hole pairs are produced from the absorption of one high-energy photon, could increase the efficiency of light absorbing devices. However, demonstrations of the effect are still scarce in photocatalytic hydrogen production. Moreover, many photocatalytic systems for overall water splitting suffer from poor charge carrier separation. Here we show that a CdTe quantum dot/vanadium-doped indium sulphide (CdTe/V-In2S3) photocatalyst has a built-in electric field and cascade energy band structure sufcient to effectively extract excitons and separate carriers, allowing MEG to be exploited for hydrogen production. We achieve a tunable energy band structure through quantum efects in CdTe and doping engineering of V-In2S3, which induces a 14-fold enhancement in the CdTe/V-In2S3 interfacial built-in electric field intensity relative to pristine CdTe/V-In2S3. We report an internal quantum efficiency of 114% at 350 nm for photocatalytic hydrogen production, demonstrating the utilization of MEG effects. The solar-to-hydrogen efficiency is 1.31%.
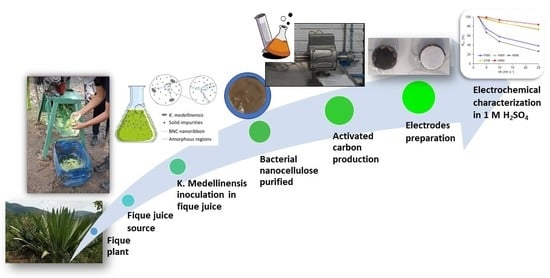
J. Villarreal-Rueda, Z. Zapata-Benabithe, L. Posada, E. Martinez, S. Herrera, S. Lopez, A. Jorge Sobrido, C. I. Castro, Polymers 2023, 15, 1760.
Abstract
This paper presents the results obtained from the chemical activation of bacterial nanocellulose (BCN) using fique juice as a culture medium. BNC activation (BNCA) was carried out with H3PO4 and KOH at activation temperatures between 500 °C to 800 °C. The materials obtained were characterized morphologically, physicochemically, superficially, and electrochemically, using scanning electron microscopy, X-ray photoelectron spectroscopy (XPS), the physisorption of gases N2 and CO2 at 77 K and 273 K, respectively, cyclic voltammetry, chronopotentiometry, and electrochemical impedance spectroscopy (EIS). The samples activated with H3PO4 presented specific surface areas (SBET) around 780 m2 g−1, while those activated with KOH values presented specific surface areas between 680 and 893 m2 g−1. The XPS analysis showed that the PXPS percentage on the surface after H3PO4 activation was 11 wt%. The energy storage capacitance values ranged between 97.5 F g−1 and 220 F g−1 by EIS in 1 M H2SO4. The samples with the best electrochemical performance were activated with KOH at 700 °C and 800 °C, mainly due to the high SBET available and the accessibility of the microporosity. The capacitance of BNCAs was mainly improved by electrostatic effects due to the SBET rather than that of pseudocapacitive ones due to the presence of phosphorus heteroatoms.
J. Gil-Rostra, J. Castillo-Seoane, Q. Guo, A. Jorge Sobrido, A. R. Gonzalez-Elipe, A. Borras, ACS Appl Mater & Interfaces 2023, 15, 9250-9262.
Abstract
A common approach for the photoelectrochemical (PEC) splitting of water relies on the application of WO3 porous electrodes sensitized with BiVO4 acting as a visible photoanode semiconductor. In this work, we propose a new architecture of photoelectrodes consisting of supported multishell nanotubes (NTs) fabricated by a soft-template approach. These NTs are formed by a concentric layered structure of indium tin oxide (ITO), WO3, and BiVO4, together with a final thin layer of cobalt phosphate (CoPi) co-catalyst. The photoelectrode manufacturing procedure is easily implementable at a large scale and successively combines the thermal evaporation of single crystalline organic nanowires (ONWs), the magnetron sputtering deposition of ITO and WO3, and the solution dripping and electrochemical deposition of, respectively, BiVO4 and CoPi, plus the annealing in air under mild conditions. The obtained NT electrodes depict a large electrochemically active surface and outperform the efficiency of equivalent planar-layered electrodes by more than one order of magnitude. A thorough electrochemical analysis of the electrodes illuminated with blue and solar lights demonstrates that the characteristics of the WO3/BiVO4 Schottky barrier heterojunction control the NT electrode efficiency, which depended on the BiVO4 outer layer thickness and the incorporation of the CoPi electrocatalyst. These results support the high potential of the proposed soft-template methodology for the large-area fabrication of highly efficient multishell ITO/WO3/BiVO4/CoPi NT electrodes for the PEC splitting of water.

L. M. Murillo-Herrera, E. S. Aguilar, M. W. Thielke, A. Jorge Sobrido, Chemistry – An Asian Journal, 2023, 18, e202201208.

Abstract
All-vanadium redox flow batteries are promising large-scale energy storage solutions to support intermittent power generation. Commercial graphite felts are among the most used materials as electrodes for these batteries due to their cheap price, high conductivity, and large surface area. However, these materials exhibit poor wettability and electrochemical activity towards vanadium redox reactions, which translates into overpotentials and lower efficiencies. Deep eutectic solvents (DES) are mixtures of Lewis acids and bases that exhibit lower melting points than their original components. Here, a DES composed of choline chloride and urea, and a DES composed of FeCl3 and NH4Cl have been employed to modify the surface of graphite felts alongside a series of re-carbonization steps. The resulting materials were compared against pristine, thermally activated, and oxidatively activated graphite felts. Our results indicated that the treatments introduced new oxygen and nitrogen functionalities to the carbonaceous surface and increased the surface area, the degree of disorder and defects in the graphitic layers of the fibres. Cyclic voltammetry studies demonstrated higher electrochemical activity towards vanadium redox reactions and electrochemical impedance spectroscopy experiments showed the modified materials exhibited significantly lower charge transfer resistances. When tested in full cell configuration the electrode modified with the urea-based DES exhibited comparable coulombic efficiencies and superior energy storage capacity retention than the thermally oxidized felt used as benchmark, suggesting that the introduction of oxygen- and nitrogen-rich functional groups had a positive effect on the overall electrochemical performance of graphite felts.
Single-Atom Iridium on Hematite Photoanodes for Solar Water Splitting: Catalyst or Spectator?
Q. Guo, Q. Zhang, R. Crespo-Otero, D. Di Tommaso, J. Tang, S. D Dimitrov, M Magdalena Titirici, X. Li, A. Jorge Sobrido, J. Amer. Chem. Soc. 2023, 145, 1686-1695.
Abstract
Single-atom catalysts (SACs) on hematite photoanodes are efficient cocatalysts to boost photoelectrochemical performance. They feature high atom utilization, remarkable activity, and distinct active sites. However, the specific role of SACs on hematite photoanodes is not fully understood yet: Do SACs behave as a catalytic site or as a spectator? By combining spectroscopic experiments and computer simulations, we demonstrate that single-atom iridium (sIr) catalysts on hematite (α-Fe2O3/sIr) photoanodes act as a true catalyst by trapping holes from hematite and providing active sites for the water oxidation reaction. In situ transient absorption spectroscopy showed a reduced number of holes and shortened hole lifetime in the presence of sIr. This was particularly evident on the second timescale, indicative of fast hole transfer and depletion toward water oxidation. Intensity-modulated photocurrent spectroscopy evidenced a faster hole transfer at the α-Fe2O3/sIr/electrolyte interface compared to that at bare α-Fe2O3. Density functional theory calculations revealed the mechanism for water oxidation using sIr as a catalytic center to be the preferred pathway as it displayed a lower onset potential than the Fe sites. X-ray photoelectron spectroscopy demonstrated that sIr introduced a mid-gap of 4d state, key to the fast hole transfer and hole depletion. These combined results provide new insights into the processes controlling solar water oxidation and the role of SACs in enhancing the catalytic performance of semiconductors in photo-assisted reactions.

Recent Advances in Ultralow‐Pt‐Loading Electrocatalysts for the Efficient Hydrogen Evolution
F. Guo, T. J. Macdonald, A. Jorge Sobrido, L. Liu, J. Feng, G. He, Adv. Sci. 2023, 231098.
Abstract
Hydrogen production from water electrolysis provides a green and sustainable route. Platinum (Pt)-based materials have been regarded as efficient electrocatalysts for the hydrogen evolution reaction (HER). However, the large-scale commercialization of Pt-based catalysts suffers from the high cost. Therefore, ultralow-Pt-loading electrocatalysts, which can reach the balance of low cost and high HER performance, have attracted much attention. In this review, representative promising synthetic strategies, including wet chemistry, annealing, electrochemistry, photochemistry, and atomic layer deposition are summarized. Further, the interaction between different electrocatalyst components (transition metals and their derivatives) and Pt is discussed. Notably, this interaction can effectively accelerate the kinetics of the HER, enhancing the catalytic activity. At last, current challenges and future perspectives are briefly discussed.
Boosting the photocatalytic performance via defect-dependent interfacial interactions from electrostatic adsorption to chemical bridging
Y. Zhang, N. Miao, X. Xin, Y. Wang, J. Zhu, P. Guo, J. Wang, A. Jorge Sobrido, M.-M. Titirici, X. Li*
Nano Energy 2022, 104, 107865
Abstract
The sluggish kinetics of interfacial electron transport and suboptimal photocatalytic stability are remaining challenges for designing efficient hetero-structured photocatalysts. Herein, we demonstrate a defect-induced interfacial interaction in the graphene oxide quantum dot/indium sulfide (GQD/In2S3) hybrid, achieving remarkable stability and efficiency. By introducing sulfur vacancies into the In2S3 structure, the interfacial electron exchange between the GQD and In2S3 drastically increases, turning the interfacial interaction from weakly electrostatic adsorption to strongly chemical bridging. The interfacial interaction transition exhibits a great advantage in kinetics of interfacial electron transport with 12.32 times increase in the internal electric field intensity and less than half of carrier transport activation energy, while preventing the sulfur leaching in In2S3 and enhancing the photocatalytic stability. Consequently, the GQD/In2S3 with chemical bridging interface exhibits a dominant photocatalytic activity, with 40.9 mmol g−1 h−1, 22.7 folds higher than the analogous materials without S vacancies, and 96.1% H2 yield retention after 100 h tests. The deep understanding of the defect-induced interfacial modulation provides an insight for the design of high-performance hybrid photocatalyst.
Can electrospun nanofibres replace traditional carbon felt electrodes in redox flow batteries?
Jorge P V Tafoya, Michael W Thielke, Gengyu Tian, Rhodri Jervis, Ana B Jorge Sobrido*
Current Opinion in Chemical Engineering 2022, 38, 100876
Electrospinning is fast finding its way as one of the preferred manufacturing techniques to process advanced materials for energy-storage applications. This is due to its remarkable advantages in terms of high versatility to produce free-standing nanofibre materials with controlled composition, porous microstructure and thickness. Among the different devices that can benefit from this technique, redox flow batteries are an emerging grid-scale energy- storage technology that uses electrodes consisting of commercially available carbon felts, cloths or papers. These materials exhibit relatively good stability and low cost. However, their activity towards relevant redox reactions is often poor, which leads to low-power densities and voltage efficiencies. Attempts to improve the electrochemical activity via thermal treatment or deposition of electrocatalytic species have produced mixed results. In addition, the microstructure and void volume are key properties that need optimisation to achieve effective mass transport. The electrodes act as porous media, providing heterogeneous interaction between the electrolyte and exposed catalytic sites. Therefore, the ability to control properties such as porous volume, specific surface area and tortuosity is highly desirable to minimise transport-limited inefficiencies and parasitic pumping losses. This opinion paper explores the potential of electrospun carbon materials to replace commercial carbons as electrodes for redox flow batteries, examining advantages, disadvantages and challenges.
Gradient Heating Epitaxial Growth Gives Well Lattice-Matched Mo2C−Mo2N Heterointerfaces that Boost Both Electrocatalytic Hydrogen Evolution and Water Vapor Splitting
Youzi Zhang, Peng Guo, Shaohui Guo, Xu Xin, Yijin Wang, Wenjing Huang, Maohuai Wang, Bowen Yang, Ana Jorge Sobrido, Jahan B Ghasemi, Jiaguo Yu, and Xuanhua Li*
Angewandte Chemie International Edition, 2022, 61, e202209703.
Abstract:
An optimized approach to producing lattice-matched heterointerfaces for electrocatalytic hydrogen evolution has not yet been reported. Herein, we present the synthesis of lattice-matched Mo2CMo2N heterostructures using a gradient heating epitaxial growth method.The well lattice-matched heterointerface of Mo2CMo2N generates near-zero hydrogen-adsorption free energy and facilitates water dissociation in acid and alkaline media. The lattice-matched Mo2CMo2N heterostructures have low overpotentials of 73 mV and 80 mV at 10 mAcm2 in acid and alkaline solutions, respectively, comparable to commercial Pt/C. A novel photothermal-electrocatalytic water vapor splitting device using the lattice-matched Mo2CMo2N heterostructure as a hydrogen evolution electrocatalyst displays a competitive cell voltage for electrocatalytic water splitting.

Photoelectrochemical Detection of Calcium Ions Based on Hematite Nanorod Sensors
Bo Zhou, Yunlu Jianf, Qian Guo, Anirbas Das, Ana Jorge Sobrido, Karin A Hing, Anatoly V Zayats and Steffi Krause*
Abstract

α-Fe2O3 (hematite) thin films have been shown to be a robust sensor substrate for photoelectrochemical imaging with good stability and high spatial resolution. Herein, one-dimensional (1D) hematite nanorods (NRs) synthesized via a simple hydrothermal method are proposed as a substrate which provides nanostructured surfaces with enhanced photocurrent responses compared to previously described hematite films, good stability, and excellent spatial resolution for potential imaging applications. The photoelectrochemical sensing capability of hematite NRs was demonstrated by a high pH sensitivity without modification. The modification of the hematite NRs with a thin poly(vinyl chloride) (PVC)-based ion-selective film allowed highly reversible amperometric detection of calcium ions with sensor materials traditionally employed in potentiometric devices.
Bioinspired and Bioderived Aqueous Electrocatalysis
Jesuś Barrio, Angus Pedersen, Silvia Favero, Hui Luo, Mengnan Wang, Saurav Ch. Sarma, Jingyu Feng, Linh Tran Thi Ngoc, Simon Kellner, Alain You Li, Ana Belén Jorge Sobrido, and Maria-Magdalena Titirici*
Chem Rev 2022 https://doi.org/10.1021/acs.chemrev.2c00429
ABSTRACT: The development of efficient and sustainable electrochemical systems able to provide clean-energy fuels and chemicals is one of the main current challenges of materials science and engineering. Over the last decades, significant advances have been made in the development of robust electrocatalysts for different reactions, with fundamental insights from both computational and experimental work. Some of the most promising systems in
the literature are based on expensive and scarce platinum-group metals; however, natural enzymes show the highest per-site catalytic activities, while their active sites are based exclusively on earth-abundant metals. Additionally, natural biomass provides a valuable feedstock for producing advanced carbonaceous materials with porous hierarchical
structures. Utilizing resources and design inspiration from nature can help create more sustainable and cost-effective strategies for manufacturing cost-effective, sustainable, and robust electrochemical materials and devices. This review spans from materials to device engineering; we initially discuss the design of carbon-based materials with bioinspired features (such as enzyme active sites), the utilization of biomass resources to construct tailored carbon materials, and their activity in aqueous electrocatalysis for water splitting, oxygen reduction, and CO2 reduction. We then delve in the applicability of bioinspired features in electrochemical devices, such as the engineering of bioinspired mass transport and electrode interfaces. Finally, we address remaining challenges, such as the stability of bioinspired active sites or the activity of metal-free carbon materials, and discuss new potential research directions that can open the gates to the implementation of bioinspired sustainable materials in electrochemical devices.

Performance and potential of porous carbons derived of electrospun metal–organic frameworks for supercapacitor applications
Journal of Energy Chemistry
Graphical abstract
A perspective on electrospinning of metal organic frameworks as a promising synthetic approach to design hierarchically porous carbon materials with application as electrode in energy storage devices.

Photoelectrochemical imaging system with high spatiotemporal resolution for visualizing dynamic cellular responses
Biosensors and Bioelectronics 2021, 113121
Photoelectrochemical imaging has great potential in the label-free investigation of cellular processes. Herein, we report a new fast photoelectrochemical imaging system (PEIS) for DC photocurrent imaging of live cells, which combines high speed with excellent lateral resolution and high photocurrent stability, which are all crucial for studying dynamic cellular processes. An analog micromirror was adopted to raster the sensor substrate, enabling high-speed imaging. α-Fe2O3 (hematite) thin films synthesized via electrodeposition were used as a robust substrate with high photocurrent and good spatial resolution. The capabilities of this system were demonstrated by monitoring cell responses to permeabilization with Triton X-100. The ability to carry out dynamic functional imaging of multiple cells simultaneously provides improved confidence in the data than could be achieved with the slower electrochemical single-cell imaging techniques described previously. When monitoring pH changes, the PEIS can achieve frame rates of 8 frames per second.

Electrospinning as a route to advanced carbon materials for selected low-temperature electrochemical devices : a review
J. Energy Chemistry 2021, 59, 492-529
Electrospinning has been proven as a highly versatile fabrication method for producing nano-structured fibres with controllable morphology, of both the fibres themselves and the void structure of the mats. Additionally, it is possible to use heteroatom doped polymers or to include catalytic precursors in the electrospinning solution to control the surface properties of the fibres. These factors make it an ideal method for the production of electrodes and flow media for a variety of electrochemical devices, enabling reduction in mass transport and activation overpotentials and therefore increasing efficiency. Moreover, the use of biomass as a polymer source has recently gained attention for the ability to embed sustainable principles in the materials of electrochemical devices, complementing their ability to allow an increase in the use of renewable electricity via their application. In this review, the historical and recent developments of electrospun materials for application in redox flow batteries, fuel cells, metal air batteries and supercapacitors are thoroughly reviewed, including an overview of the electrospinning process and a guide to best practice. Finally, we provide an outlook for the emerging use of this process in the field of electrochemical energy devices with the hope that the combination of tailored microstructure, surface functionality and computer modelling will herald a new era of bespoke functional materials that can significantly improve the performance of the devices in which they are used.

The Role of Carbon Dots – Derived Underlayer in Hematite Photoanodes
Nanoscale 2020, 12, 20220-20229
Hematite is a promising candidate as photoanode for solar-driven water splitting, with a theoretically predicted maximum solar-to-hydrogen conversion efficiency of ∼16%. However, the interfacial charge transfer and recombination greatly limits its activity for photoelectrochemical water splitting. Carbon dots exhibit great potential in photoelectrochemical water splitting for solar to hydrogen conversion as photosensitisers and co-catalysts. Here we developed a novel carbon underlayer from low-cost and environmental-friendly carbon dots through a facile hydrothermal process, introduced between the fluorine-doped tin oxide conducting substrate and hematite photoanodes. This led to a remarkable enhancement in the photocurrent density. Owing to the triple functional role of carbon dots underlayer in improving the interfacial properties of FTO/hematite and providing carbon source for the overlayer as well as the change in the iron oxidation state, the bulk and interfacial charge transfer dynamics of hematite are significantly enhanced, and consequently led to a remarkable enhancement in the photocurrent density. The results revealed a substantial improvement in the charge transfer rate, yielding a charge transfer efficiency of up to 80% at 1.25 V vs. RHE. In addition, a significant enhancement in the lifetime of photogenerated electrons and an increased carrier density were observed for the hematite photoanodes modified with a carbon underlayer, confirming that the use of sustainable carbon nanomaterials is an effective strategy to boost the photoelectrochemical performance of semiconductors for energy conversion.
Heat Diffusion‐Induced Gradient Energy Level in Multishell Bisulfides for Highly Efficient Photocatalytic Hydrogen Production
Insufficient light absorption and low carrier separation/transfer efficiency constitute two key issues that hinder the development of efficient photocatalytic hydrogen production. Here, multishell ZnS/CoS2 bisulfide microspheres with gradient distribution of Zn based on the heat diffusion theory are designed. The Zn distribution can be adjusted by regulating the heating rate and manipulating the diffusion coefficients of the different elements conforming the multishell photocatalyst. Because of the unique structure, a gradient energy level is created from the core to the exterior of the multishell microspheres, which effectively facilitates the exciton separation and electron transfer. In addition, stronger light absorption and larger specific surface area have been achieved in the multishell ZnS/CoS2 photocatalysts. As a result, the multishell ZnS/CoS2 microspheres with gradient distribution of Zn exhibit a remarkable hydrogen production rate of 8001 µmol g−1 h−1, which is 3.5 times higher than that of the normal multishell ZnS/CoS2 particles with well‐distributed Zn and 11.3 times higher than that of the mixed nonshell ZnS and CoS2 particles. This work demonstrates for the first time that controlling the diffusion rate of the different elements in the semiconductor is an effective route to simultaneously regulate morphology and structure to design highly efficient photocatalysts.
-
Adv. Funct. Mater. 2020, 2003035
A deeper understanding of the water‐splitting hydrogen evolution reaction (HER) mechanism during photocatalytic processes is crucial for the rational design of efficient photocatalysts. In particular, the HER mechanism promoted by multielement hybrid structures remains extremely challenging and elusive. Herein, an in situ photoelectrochemical/Raman measurement system is employed to monitor the HER mechanism of hybrid nanostructures under realistic working conditions via operando Raman spectra and linear‐sweep voltammetry curves. As a proof of concept, tunable composition transition metal dichalcogenides MoS2xSe2(1−x) nanosheets are used as a model photocatalyst to unveil the corresponding photocatalytic mechanism. The spectroscopic studies reveal that hydrogen atoms can be adsorbed to active sulfur and selenium atoms via intermediate species formed during the photocatalytic process. More importantly, the studies demonstrate that an exponential relationship exists between the number of reactive electrons and the Raman intensity of intermediate species, which can serve as a guideline to directly evaluate the HER performance in photocatalysts by comparing the Raman intensities of the intermediate species. As a simple, intuitive, and general analytical method, the designed operando Raman measurement approach provides a new tool for elucidating catalytic reaction mechanisms in a realistic and complex environment; and strategically improving H2 production performance of multielement photocatalysts.
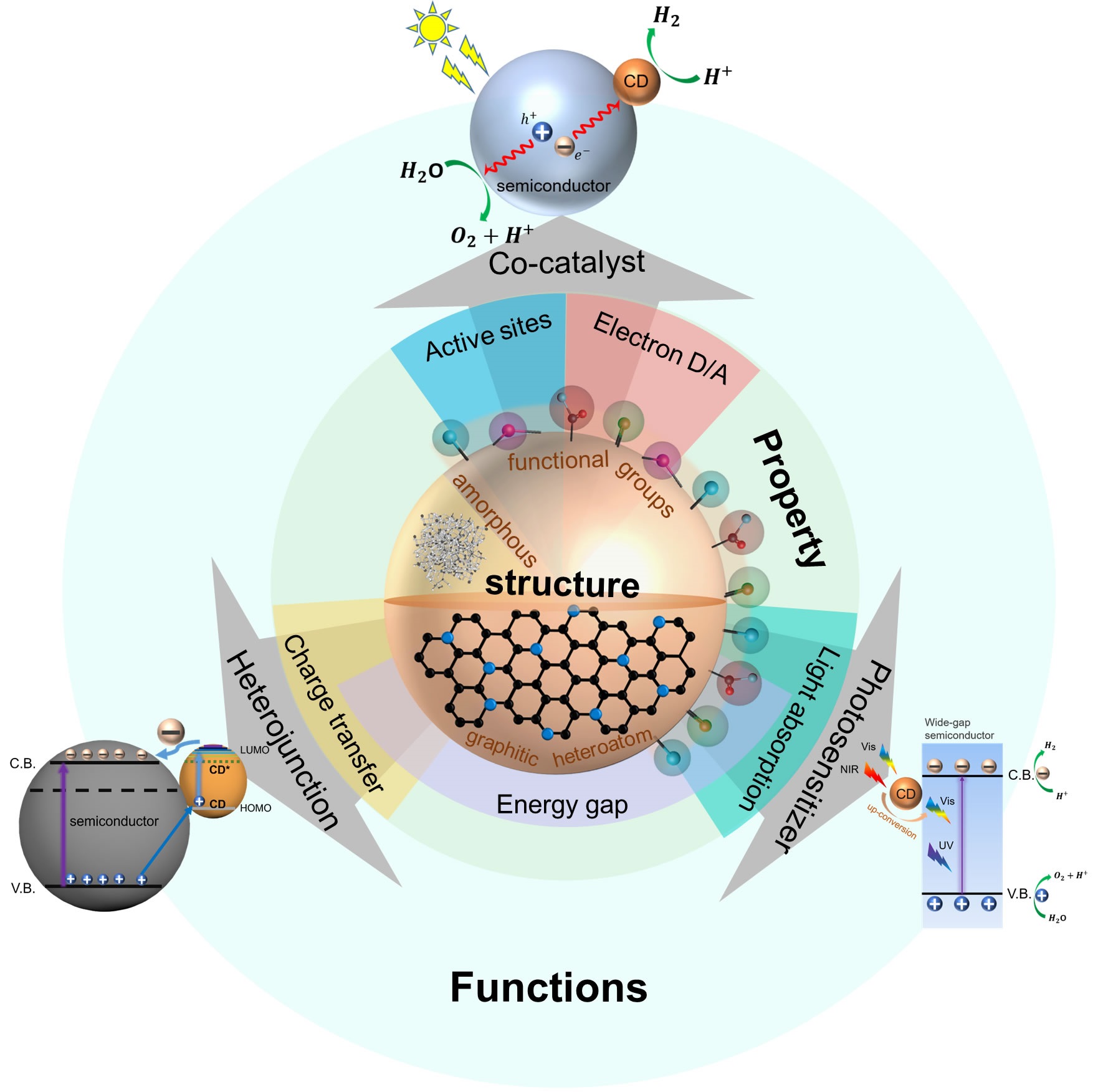
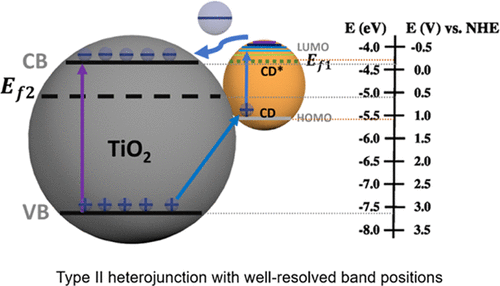
Carbon Dots in Solar-to-Hydrogen Conversion
H. Luo, Q. Guo, P. Szilagyi, A. Belen Jorge, M. Titirici, Trends in Chemistry 2020.
Solar hydrogen production from catalytic water splitting is one of the many options available to help generate clean power and alleviate the threatening environmental concerns stemming from the use of fossil fuels. During the past decade, carbon dots (CDs) have shown great potential in their application for solar-driven hydrogen production owing to their exceptional photophysical and electrical properties derived from their sp2/sp3 hybridized core structure and rich surface functionality. In this review, we correlate the structural features of CDs with their optical and electronic properties and evaluate key properties for efficient solar energy-conversion applications with an emphasis on photocatalysis and photoelectrocatalysis, to shed some light on designing high performance CD-based photosystems.
-
3D Carbon Materials for Efficient Oxygen and Hydrogen Electrocatalysis
- Ana Belen Jorge, Rhodri Jervis, Arun Prakash Periasamy, Mo Qiao, Jingyu Feng, Linh Ngoc Tran, Maria‐Magdalena Titirici, Adv. Energy Mater. 2020, 10, 2070047.
- Abstract
- Sustainable energy production at an acceptable cost is key for its widespread application. At present, noble metals and metal oxides are the most widely used for electrocatalysis, but they suffer from low selectivity, poor durability, and scarcity. Because of this, metal‐free carbons have become the subject of great interest as promising alternative electrocatalysts for energy conversion and storage devices, and remarkable progress has been accomplished in the advance of metal‐free carbons as electrocatalysts for renewable energy technologies. Particularly interesting are 3D porous carbon architectures, which exhibit outstanding features for electrocatalysis applications, including broad range of active sites, interconnected porosity, high conductivity, and mechanical stability. This review summarizes the latest advances in 3D porous carbon structures for oxygen and hydrogen electrocatalysis. The structure–performance relationship of these materials is consequently rationalized and perspectives on creating more efficient 3D carbon electrocatalysts are suggested.

- Nitrogen-doped Carbon Dots/TiO2 Nanoparticle Composites for Photoelectrochemical Water Oxidation
- Hui Luo, Stoichko D Dimitrov, Matyas Daboczi, Ji-Seon Kim, Qian Guo, Yuanxing Fang, Marc-Antoine Stoeckel, Paolo Samorì, Oliver Fenwick, Ana Belen Jorge Sobrido, Xinchen Wang, Maria-Magdalena Titirici, ACS Applied Nano Mater. 2020, 3, 3317-3381.
- Abstract
- Carbon dots on photoactive semiconductor nanomaterials have represented an effective strategy for enhancing their photoelectrochemical (PEC) activity. By carefully designing and manipulating a carbon dot/support composite, a high photocurrent could be obtained. Currently, there is not much fundamental understanding of how the interaction between such materials can facilitate the reaction process. This hinders the wide applicability of PEC devices. To address this need of improving the fundamental understanding of the carbon dots/semiconductor nanocomposite, we have taken the TiO2 case as a model semiconductor system with nitrogen-doped carbon dots (NCDs). We present here with in-depth investigation of the structural hybridization and energy transitions in the NCDs/TiO2 photoelectrode via high-resolution scanning transmission microscopy (HR-STEM), electron energy loss spectroscopy (EELS), UV–vis absorption, electrochemical impedance spectroscopy (EIS), Mott–Schottky (M–S), time-correlated single-photon counting (TCSPC), and ultraviolet photoelectron spectroscopy (UPS), which shed some light on the charge-transfer process at the carbon dots and TiO2 interface. We show that N doping in carbon dots can effectively prolong the carrier lifetime, and the hybridization of NCDs and TiO2 is able not only to extend TiO2 light response into the visible range but also to form a heterojunction at the NCDs/TiO2 interface with a properly aligned band structure that allows a spatial separation of the charges. This work is arguably the first to report the direct probing of the band positions of the carbon dot–TiO2 nanoparticle composite in a PEC system for understanding the energy-transfer mechanism, demonstrating the favorable role of NCDs in the photocurrent response of TiO2 for the water oxidation process. This study reveals the importance of combining structural, photophysical, and electrochemical experiments to develop a comprehensive understanding of the nanoscale charge-transfer processes between the carbon dots and their catalyst supports.
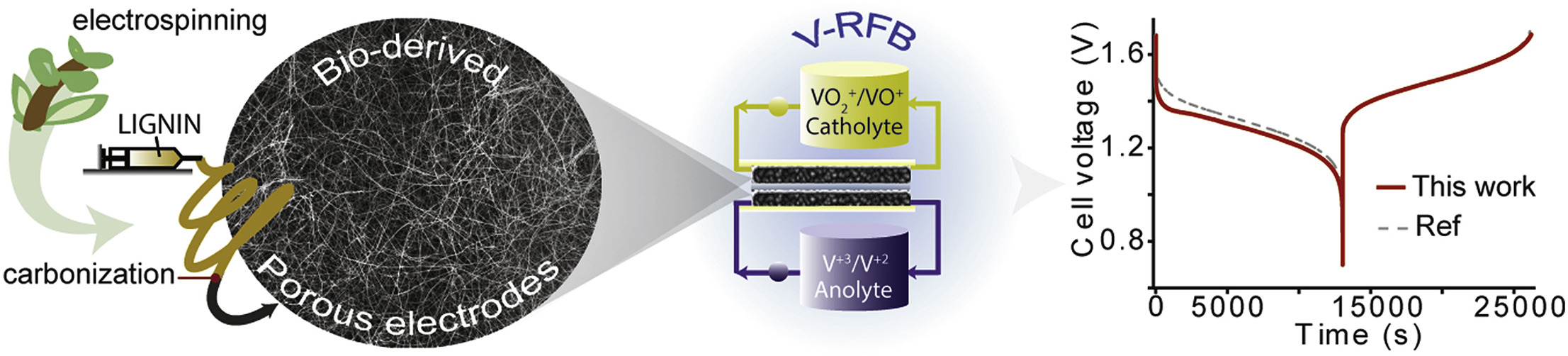
- Lignin-derived electrospun freestanding carbons as alternative electrodes for redox flow batteries
- Maria Crespo Ribadeneyra, Lia Grogan, Heather Au, Philipp Schlee, Servann Herou, Tobias Neville, Patrick L Cullen, Matt DR Kok, Omid Hosseinaei, Sverker Danielsson, Per Tomani, MM Titirici, Daniel JL Brett, Paul R Shearing, Rhodri Jervis, Ana Belen Jorge, Carbon 2020, 157, 847-856.
- Abstract
- Redox flow batteries represent a remarkable alternative for grid-scale energy storage. They commonly employ carbon felts or carbon papers, which suffer from low activity towards the redox reactions involved, leading to poor performance. Here we propose the use of electrospun freestanding carbon materials derived from lignin as alternative sustainable electrodes for all-vanadium flow batteries. The lignin-derived carbon electrospun mats exhibited a higher activity towards the VO2+/VO2+ reaction than commercial carbon papers when tested in a three-electrode electrochemical cell (or half-cell), which we attribute to the higher surface area and higher amount of oxygen functional groups at the surface. The electrospun carbon electrodes also showed performance comparable to commercial carbon papers, when tested in a full cell configuration. The modification of the surface chemistry with the addition of phosphorous produced different effect in both samples, which needs further investigation. This work demonstrates for the first time the application of sustainably produced electrospun lignin-derived carbon electrodes in a redox flow cell, with comparable performance to commercial materials and establishes the great potential of biomass-derived carbons in energy devices.
- Manipulating the optical properties of carbon dots via fine tuning their structural features
- Hui Luo, Nikolaos Papaioannou, Enrico Salvadori, Maxie M Roessler, Gereon Ploenes, Ernst RH van Eck, Liviu C Tanase, Jingyu Feng, Yiwei Sun, Yan Yang, Mohsen Danaie, Ana Belen Jorge, Andrei Sapelkin, James Durrant, Stoichko D Dimitrov, Maria‐Magdalena Titirici, ChemSusChem 2020 , 12, 4432-4441.
- Abstract
As a new class of sustainable carbon material, “carbon dots” is an umbrella term covering many types of materials. Herein, a broad range of techniques was used to develop the understanding of hydrothermally synthesized carbon dots, and it is shown how fine‐tuning the structural features by simple reduction/oxidation reactions can drastically affect their excited‐state properties. Structural and spectroscopic studies found that photoluminescence originates from direct excitation of localized fluorophores involving oxygen functional groups, whereas excitation at graphene‐like features leads to ultrafast phonon‐assisted relaxation and largely quenches the fluorescent quantum yields. This is arguably the first study to identify the dynamics of photoluminescence including Stokes shift and allow the relaxation pathways in these carbon dots to be fully resolved. This comprehensive investigation sheds light on how understanding the excited‐state relaxation processes in different carbon structures is crucial for tuning the optical properties for any potential commercial applications.

- Performance of Candlenut Shell (Alleuretus moluccana) Based Supercapacitor Electrode with Acid Electrolytes and Their Salts
- M Zakir, H Kasim, I Raya, Y Lamba, A Belen Jorge, IOP Conference Series: Materials Science and Engineering 2019, 619, 012042.
- Abstract
- Candlenut shell was used as the precursor for activated carbon-based electrodes which prepared through two stages, namely carbonization and activation. The surface of the activated carbon was modified with HNO3. Characterization of carbons and activated carbon was performed by SEM, BET, FT-IR test and capacitance analysis using Cyclic Voltammetry. The result of SEM analysis shows that carbon pore formation has occurred after activation with H3PO4. The surface area of carbon and activated carbon determined by the BET method is 125,828 m2/g and 142,435 m2/g, respectively. The results of FTIR and Boehm titration analysis showed an increase in oxygen-containing functional groups after modification with HNO3. Based on the results of the analysis using Cyclic Voltammetry obtained the highest specific capacitance of unmodified activated carbon is 145.49 mF/g in H2SO4 0.5M electrolyte. In addition, the highest specific capacitance of activated carbon modified with HNO3 is 155.92 mF/g obtained in HCl 0.1 M electrolyte. In the same condition, the contribution of salt electrolyte (Na2SO4 0.5 M and 0.1 M) in the specific capacitance of candlenut shell based supercapacitor electrodes is a little bit lower compared to the acid electrolytes.
- High-power nitrided TiO2 carbon felt as the negative electrode for all-vanadium redox flow batteries
-
J Vázquez-Galván, C Flox, JR Jervis, AB Jorge, PR Shearing, JR Morante, Carbon 2019, 148, 91-104
Abstract
This work describes the design of an electrode with enhanced performance applied to all-vanadium redox flow batteries (VRFBs). This new electrode consists of a structural porous carbon felt decorated with TiO2 rutile nanoparticles, which has been nitrided using ammonolysis at 900 °C. An outstanding charge and mass transfer over the electrode-electrolyte interface was observed as a consequence of the synergetic effect of N- and O-functionalization over carbon felt (CF) and the partial formation of TiN (metallic conductor) phase. Moreover, this material has not only improved in terms of catalysis towards the V3+/V2+ redox reaction (k0 = 1.6 × 10−3 cm s−1), but also inhibited the hydrogen evolution reaction (HER), which is one of the main causes of imbalances that lead to battery failure. This led to an impressive high-power peak output value up to 700 mW cm−2, as well as work at high current density in galvanostatic conditions (i.e. 150 mA cm−2), exhibiting low ohmic losses (overpotential) and great redox single cell reversibility, with a superior energy efficiency of 71%. An inexpensive, earth abundant and scalable synthesis method to boost VRFBs technology based on nitrided CF@TiO2 is presented, being able to overcome certain constrains, and therefore to achieve high energy and power densities.

-
Free-standing supercapacitors from Kraft lignin nanofibers with remarkable volumetric energy density
- Philipp Schlee, Servann Herou, Rhodri Jervis, Paul R Shearing, Dan JL Brett, Darren Baker, Omid Hosseinaei, Per Tomani, M Mangir Murshed, Yaomin Li, María José Mostazo-López, Diego Cazorla-Amorós, Ana Belen Jorge Sobrido, Maria-Magdalena Titirici, Chem. Sci. 2019, 10, 2980-2988.
Abstract
We have discovered a very simple method to address the challenge associated with the low volumetric energy density of free-standing carbon nanofiber electrodes for supercapacitors by electrospinning Kraft lignin in the presence of an oxidizing salt (NaNO3) and subsequent carbonization in a reducing atmosphere. The presence of the oxidative salt decreases the diameter of the resulting carbon nanofibers doubling their packing density from 0.51 to 1.03 mg cm−2 and hence doubling the volumetric energy density. At the same time, the oxidative NaNO3 salt eletrospun and carbonized together with lignin dissolved in NaOH acts as a template to increase the microporosity, thus contributing to a good gravimetric energy density. By simply adjusting the process parameters (amount of oxidizing/reducing agent), the gravimetric and volumetric energy density of the resulting lignin free-standing carbon nanofiber electrodes can be carefully tailored to fit specific power to energy demands. The areal capacitance increased from 147 mF cm−2 in the absence of NaNO3 to 350 mF cm−2 with NaNO3 translating into a volumetric energy density increase from 949 μW h cm−3 without NaNO3 to 2245 μW h cm−3 with NaNO3. Meanwhile, the gravimetric capacitance also increased from 151 F g−1 without to 192 F g−1 with NaNO3.
- Edge-Rich MoS2 Grown on Edge-Oriented Three-dimensional Graphene Glass for High-Performance Hydrogen Evolution
- Xuanhua Li, Shaohui Guo, Wei Li, Xingang Ren, Jie Su, Qiang Song, Ana Jorge Sobrido, Bingqing Wei, Nano Energy 2019, 57, 388-397.
- Abstract – The more exposures of the photocatalytically active sites are one of the essential elements to achieve high photocatalytic efficiency. Through the architecture designs, we have proposed an edge-rich MoS2 nanoarray grown on an edge-oriented three-dimensional (3D) graphene (termed as the 3D-graphene/E-MoS2) via chemical vapor deposition. Unlike the two-dimensional (2D) graphene, the highly conductive and transparent 3D graphene film has been grown at oblique angles on glass (i.e., a graphene glass), providing the large exposed surface area for the loading of more photocatalysts. Then, the abundant photocatalytically active sites can be achieved in the subsequently deposited edge-rich MoS2 nanoarrays, which are significantly beneficial for photocatalytic hydrogen production. The theoretical and experimental studies have revealed the new finding in the substantial improvements of both optical and electrical properties based on the geometrically designed 3D-graphene/E-MoS2 structures. Optically, the excellent light absorption (wavelength range: 300–800 nm) is observed, which is attributed to the favorable energy band for the efficient charge transfer between the electronically interconnected graphene and MoS2, and orientation of the MoS2 crystal face array. Electrically, the edge-rich MoS2 grown on the edge-oriented 3D graphene glass can achieve the optimized charge transport along the 2D vector plane from MoS2 layers to graphene. Consequently, the new hybrid nanostructures exhibit excellent performance as an effective photocatalyst for hydrogen generation from photocatalytic water splitting. The measured hydrogen evolution rate (2232.7 μmol/g/h) under white-light illumination is one of the highest among those photocatalysts reported to date.
8.Mohamed Karmaoui, Ana Belen Jorge, Paul F McMillan, Abil E Aliev, Robert C Pullar, João António Labrincha, David Maria Tobaldi, ACS Omega 2018, 3, 13227-1323
Abstract –
Because of its electrically conducting properties combined with excellent thermal stability and transparency throughout the visible spectrum, tin oxide (SnO2) is extremely attractive as a transparent conducting material for applications in low-emission window coatings and solar cells, as well as in lithium-ion batteries and gas sensors. It is also an important catalyst and catalyst support for oxidation reactions. Here, we describe a novel nonaqueous sol–gel synthesis approach to produce tin oxide nanoparticles (NPs) with a low NP size dispersion. The success of this method lies in the nonhydrolytic pathway that involves the reaction between tin chloride and an oxygen donor, 1-hexanol, without the need for a surfactant or subsequent thermal treatment. This one-pot procedure is carried out at relatively low temperatures in the 160–260 °C range, compatible with coating processes on flexible plastic supports. The NP size distribution, shape, and dislocation density were studied by powder X-ray powder diffraction analyzed using the method of whole powder pattern modeling, as well as high-resolution transmission electron microscopy. The SnO2 NPs were determined to have particle sizes between 3.4 and 7.7 nm. The reaction products were characterized using liquid-state 13C and 1H nuclear magnetic resonance (NMR) that confirmed the formation of dihexyl ether and 1-chlorohexane. The NPs were studied by a combination of 13C, 1H, and 119Sn solid-state NMR as well as Fourier transform infrared (FTIR) and Raman spectroscopy. The 13C SSNMR, FTIR, and Raman data showed the presence of organic species derived from the 1-hexanol reactant remaining within the samples. The optical absorption, studied using UV–visible spectroscopy, indicated that the band gap (Eg) shifted systematically to lower energy with decreasing NP sizes. This unusual result could be due to mechanical strains present within the smallest NPs perhaps associated with the organic ligands decorating the NP surface. As the size increased, we observed a correlation with an increased density of screw dislocations present within the NPs that could indicate relaxation of the stress. We suggest that this could provide a useful method for band gap control within SnO2 NPs in the absence of chemical dopants.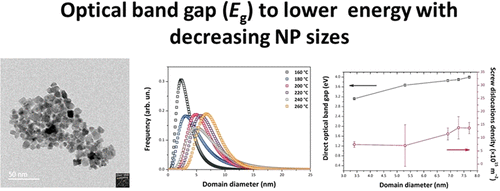
Dina Ibrahim Abouelamaiem, María José Mostazo-López, Guanjie He, Drasti Patel, Tobias P Neville, Ivan P Parkin, Dolores Lozano-Castelló, Emilia Morallón, Diego Cazorla-Amorós, Ana Belen Jorge, Rongfang Wang, Shan Ji, Maria-Magdalena Titirici, Paul R Shearing, Daniel JL Brett, J. Energy Storage 2018, 19, 337-347.
Abstract
Activated carbons, with different surface chemistry and porous textures, were used to study the mechanism of electrochemical hydrogen and oxygen evolution in supercapacitor devices. Cellulose precursor materials were activated with different potassium hydroxide (KOH) ratios, and the electrochemical behaviour was studied in 6 M KOH electrolyte. In situ Raman spectra were collected to obtain the structural changes of the activated carbons under severe electrochemical oxidation and reduction conditions, and the obtained data were correlated to the cyclic voltammograms obtained at high anodic and cathodic potentials. Carbon-hydrogen bonds were detected for the materials activated at high KOH ratios, which form reversibly under cathodic conditions. The influence of the specific surface area, narrow microporosity and functional groups in the carbon electrodes on their chemical stability and hydrogen capture mechanism in supercapacitor applications has been revealed.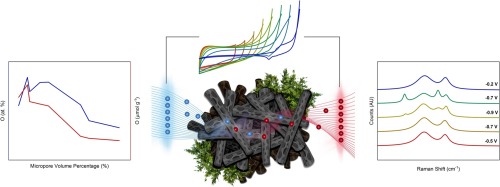
Dina Ibrahim Abouelamaiem, Guanjie He, Tobias P Neville, Drasti Patel, Shan Ji, Rongfang Wang, Ivan P Parkin, Ana Belen Jorge, Maria-Magdalena Titirici, Paul R Shearing, Daniel JL Brett, Electrochim. Acta 2018, 284, 597-608.
Abstract – This work considers the relationship between the morphology of porous carbon materials used for supercapacitors and the electrochemical impedance spectroscopy (EIS) response. EIS is a powerful tool that can be used to study the porous 3-dimensional electrode behavior in different electrochemical systems. Porous carbons prepared by treatment of cellulose with different compositions of potassium hydroxide (KOH) were used as model systems to investigate the form vs. electrochemical function relationship. A simple equivalent circuit that represents the electrochemical impedance behavior over a wide range of frequencies was designed. The associated impedances with the bulk electrolyte, Faradaic electrode processes and different pore size ranges were investigated using a truncated version of the standard transmission line model. The analysis considers the requirements of porous materials as electrodes in supercapacitor applications, reasons for their non-ideal performance and the concept of ‘best capacitance’ behavior in different frequency ranges.
Dina Ibrahim Abouelamaiem, Lara Rasha, Guanjie He, Tobias P Neville, Jason Millichamp, Tom J Mason, Ana Belen Jorge, Ivan P Parkin, Maria-Magdalena Titirici, Rongfang Wang, Shan Ji, Paul R Shearing, Daniel JL Brett, J. Energy Storage 2018, 19, 28-34.
Abstract – Physically integrated energy storage devices are gaining increasing interest due to the rapid development of flexible, wearable and portable electronics technology. For the first time, supercapacitor components have been integrated into a printed circuit board (PCB) construct. This proof-of-concept study paves the way for integrating supercapacitors into power electronics devices and hybridising with PCB fuel cells. Commercial Norit activated carbon (NAC) was used as the electrode material and was tested in two types of electrolytes, sodium sulfate (Na2SO4) aqueous electrolyte, and Na2SO4-polyvinyl alcohol (Na2SO4-PVA) gel electrolyte. Electrochemical measurements compare the SC-PCBs to standard two-electrode button-cell supercapacitors. A volumetric energy density of 0.56 mW h cm−3 at a power density of 26 mW cm−3 was obtained in the solid-state SC-PCB system, which is over twice the values acquired in the standard cell configuration. This is due to the removal of bulky components in the standard cell, and/or decreased thickness of the overall device, and thus a decrease in the total volume of the SC-PCB configuration. The results show great potential for embedding supercapacitors into PCBs for a broad range of applications. In addition, further advantages can be realised through close physical integration with other PCB-based electrochemical power systems such as fuel cells.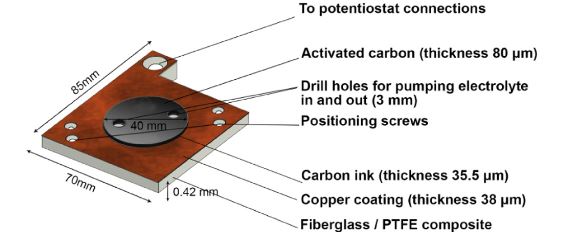
-
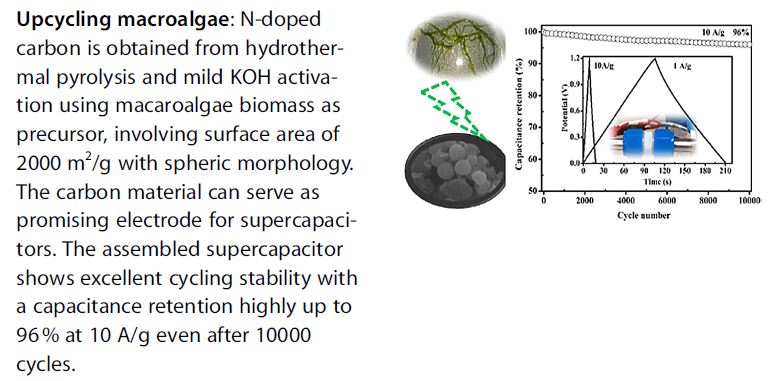
High performance N‐doped carbon electrodes obtained via hydrothermal carbonization of macroalgae for supercapacitor applications
-
M. Ren, Z. Jia, Z. Tian, D. Lopez, J. Cai, M. M. Titirici, A. Belen Jorge, ChemElectroChem 5, 2686-2693
Abstract
The conversion of bio‐waste into useful porous carbons constitutes a very attractive approach to contribute to the development of sustainable energy economy, even more as they can be used in energy storage devices. Here we report the synthesis of N‐doped carbons from hydrothermal carbonization of macroalgae, Enteromorpha prolifera (EP), followed by a mild KOH activation step. The obtained N‐doped carbon microspheres exhibited surface areas of up to ~2000 m2/g with N‐loadings varied in the ranges of 1.4~2.9 at%. By modifying activation temperature, we were able to tune surface chemistry and porosity, achieving excellent control of their properties. The specific capacitance reached value of up to 200 F/g at 1 A/g in 6M KOH, for the sample (AHC‐700) obtained at activation temperature of 700 ºC. The as‐assembled symmetric supercapacitor using the sample (AHC‐800) activated at 800 ºC as the electrodes exhibited superior cycling stability with capacitance retention of up to 96% at 10 A/g even after 10,000 cycles, constituting the highest reported so far for biomass‐derived carbon electrodes. These results show the great potential of N‐doped carbons as electrodes for supercapacitors and confirm the excellent electrochemical properties of biomass‐derived carbons in energy storage technologies.
- Carbon Nitride Materials as Efficient Catalyst Supports for Proton Exchange Membrane Water Electrolyzers,
- AB Jorge, I Dedigama, TS Miller, P Shearing, DJL Brett, PF McMillan, Nanomaterials, 2018, 8,
Abstract
Carbon nitride materials with graphitic to polymeric structures (gCNH) were investigated as catalyst supports for the proton exchange membrane (PEM) water electrolyzers using IrO₂ nanoparticles as oxygen evolution electrocatalyst. Here, the performance of IrO₂nan o particles formed and deposited in situ onto carbon nitride support for PEM water electrolysis was explored based on previous preliminary studies conducted in related systems. The results revealed that this preparation route catalyzed the decomposition of the carbon nitride to form a material with much lower N content. This resulted in a significantenhancement of the performance of the gCNH-IrO₂ (or N-doped C-IrO₂) electrocatalyst that was likely attributed to higher electrical conductivity of the N-doped carbon support.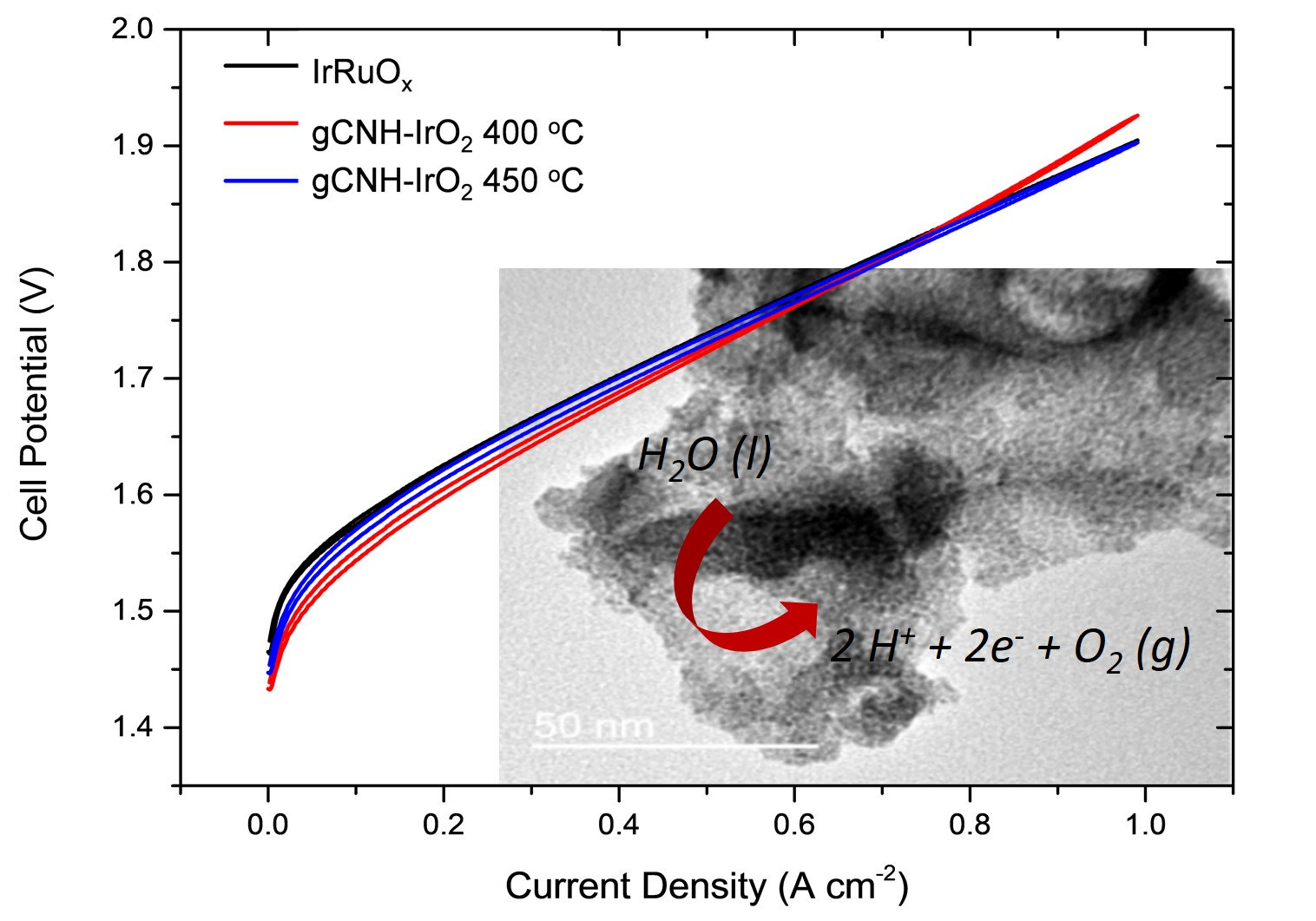
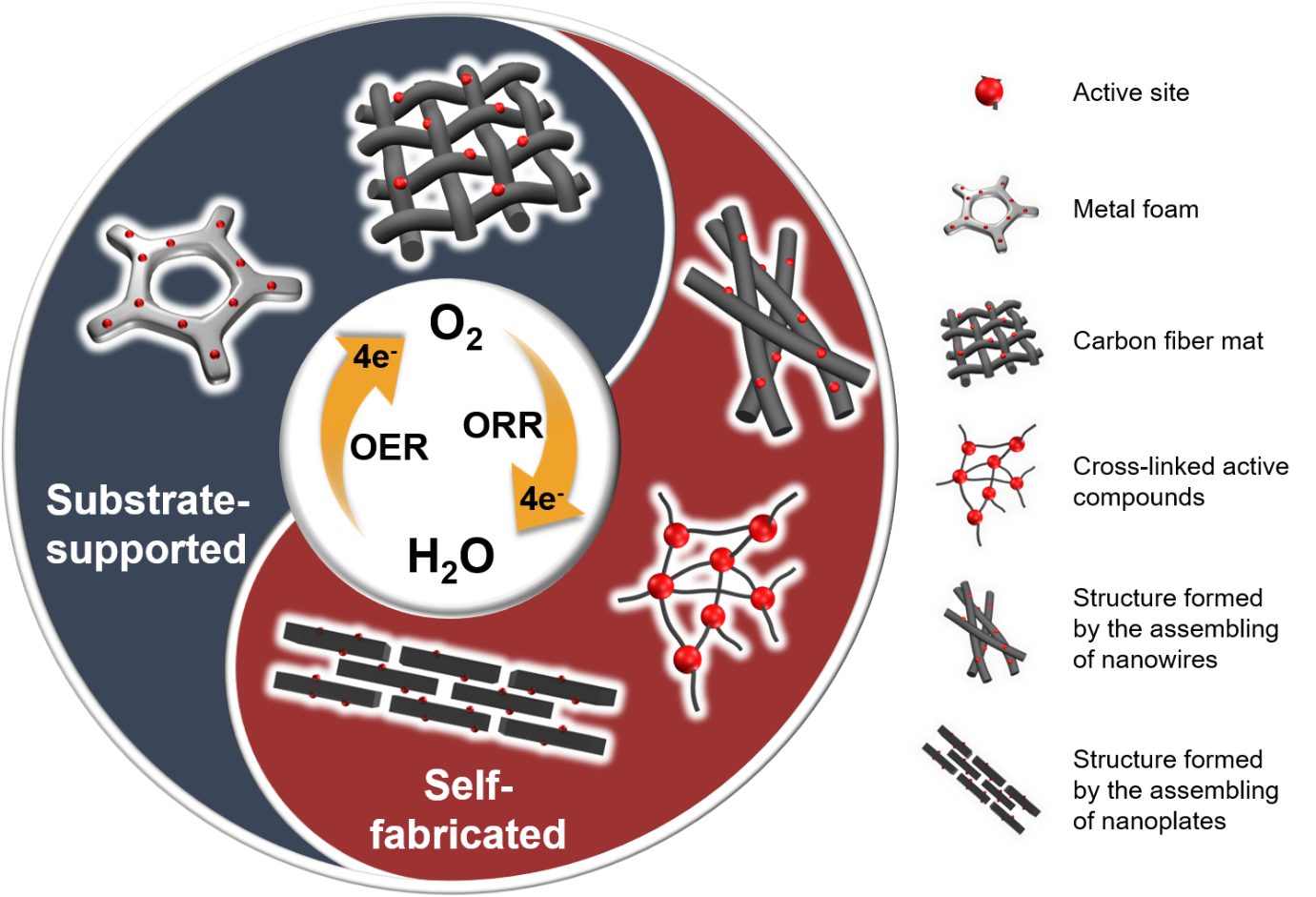
- Freestanding Non-Precious Metal Electrocatalysts for Oxygen Evolution and Reduction Reactions,
- H-F. Wang, R. Chen, J. Feng, M. Qiao, S. Doczcezczo, Q. Zhang, M. M. Titirici, A. Belen Jorge, ChemElectroChem, 2018, DOI: 10.1002/celc.201800292R1.
Abstract
Carbon and transition metals have emerged as promising candidates for many energy storage and conversion devices. They facilitate charge transfer reactions whilst showing a good stability. These materials, fabricated as freestanding electrodes pose the potential of simplified electrode manufacturing procedures whilst demonstrating excellent electrocatalytic, mechanical, and structural properties, resulting from interconnected (via chemical or van der Waals force bonded) network structures. In such freestanding configuration, the lack of a binder leads to a better conductivity, ease in the manufacturing processing, and allows a lower catalyst mass loading, all of which lead to obvious benefits. This Minireview summarizes different fabrication techniques of freestanding non‐precious‐metal oxygen electrocatalysts along with their performance towards the oxygen evolution reaction (OER) and/or oxygen reduction reaction (ORR). Here, we discuss electrocatalysts produced by using freestanding substrates and those obtained through the self‐assembly of different precursors. The advantages of using freestanding versus non‐freestanding configurations are also pondered. Challenges and perspectives for freestanding electrocatalysts are presented at the end of the Minireview as a guideline for future studies in the field. This work is expected to serve as inspiration for science colleagues to develop further studies into design, processing and testing strategies of freestanding low‐cost oxygen electrocatalysts.
- Determination of specific capacitance of modified candlenut shell based carbon as electrode material for supercapacitor,
- Zakir, P. Budi, I. Raya, A. Karim, R. Wulandarai, A. Belen Jorge, J. Phys.: Conf. Ser. 2018, 979, 012024 (1-7).
Abstract
Surface modification of candlenut shell carbon (CSC) using three chemicals: nitric acid (HNO3), hydrogen peroxide (H2O2), and sulfuric acid (H2SO4) has been carried out. Activation of CSC was performed using H3PO4 solution with different ratio between CSC and activator. Carbon surface area was determined by methylene blue adsorption method. Surface characterization was performed using FTIR spectroscopy and Boehm titration method. Specific capacitance of electrode prepared from CSAC (candlenuts shell activated carbon) materials was quantified by Cyclic Voltammetry (CV) measurement. The surface area before and after activation are 105,127 m2/g, 112,488 m2/g, 124,190 m2/g, and 135,167 m2/g, respectively. Surface modification of CSAC showed the improvement in the chemical functionality of CSAC surface. Analyses using FTIR spectroscopy and Boehm titration showed that modifications with HNO3, H2SO4 and H2O2 on the surface of the CSAC increased the number of oxygen functional groups. As a consequence, the specific capacitance of CSAC modified with 65% HNO3 attained the highest value (127 μF/g). There is an incredible increase by a factor of 298% from electrode which was constructed with un-modified CSAC material. This increase correlates to the largest number of oxygen functional groups of CSAC modified with nitric acid (HNO3).
- Synergistic Relationship between the Three-Dimensional Nanostructure and Electrochemical Performance in Biocarbon Supercapacitor Electrode Materials,
- Dina Ibrahim Abouelamaiem, Guanjie He, Ivan P Parkin, Tobias Neville, A Belen Jorge, Shan Ji, Rongfang Wang, Magdalena Titirici, Dan Shearing, Paul and Brett, Sustainable Energy & Fuels, just accepted, 2018. doi: 10.1039/C7SE00519A.
Abstract
A novel study presented herein correlates the multidimensional morphology with the electrochemical performance of activated bio-carbon materials, for supercapacitor devices over multiple length scales. The optimization of the potassium hydroxide (KOH) / cellulose ratio for supercapacitor electrode materials is related to morphological characteristics and corresponding electrochemical performance, as described in terms of porosity, specific surface area, specific capacitance and electrochemical impedance. KOH / cellulose samples with ratios 0.5:1 and 1:1 exhibited the best performance, characterized by a hierarchal porous network structure, high surface area and low cell resistance. Compared with the rest of the manufactured samples and commercial activated carbons, Ketjen Black (KB), Norit activated carbon (NAC) and bead-shaped activated carbon (BAC), the former two samples showed better results in three-electrode systems and coin cells, with specific gravimetric capacitances as high as 187 F g-1 at a current density of 1 A g-1. The high performance is attributed to the morphology of the samples that constituted a combination of micro-, meso- and macro-porosity which consequently gave high specific surface area, high porosity, low cell resistance and high specific capacitance. This further corroborates the structure-performance relationship observed in the author’s model KOH/cellulose system, highlighting that the work can be extended to other similar systems. It is clear that the three-dimensional nanostructure of a material must be understood in its entirety in order to optimize the electrochemical performance.
- Biomass-derived electrodes for flexible supercapacitors.
- Servann Herou, Philipp Schlee, Ana Belen Jorge, Magdalena Titirici, Current Opinions in Green and Sustainable Chemistry, 2017, 9, 18-24.
Abstract
At present, supercapacitors constitute, along with batteries, one of the most promising electrochemical energy storage technology. The recent emerging generation of bendable portable electronic devices has boosted the research of new materials, new processing techniques and new designs that can meet the demands in terms of mechanical stability upon bending or stretching, without compromising their electrochemical performance, at an acceptable cost. Among all the electrode materials currently explored, biomass-derived carbons hold a great potential, due to their low-cost, easy processing techniques, stability and versatility. Here we introduce the range of renewable precursors available and current state-of-the-art performances, and explore the challenges regarding flexibility and sustainability.

- The Importance of Using Alkaline Ionomer Binders for Screening Electrocatalysts in Alkaline Electrolyte, Rhodri Jervis, Noramalina Mansor, A. Belen Jorge, Simon Jones, Christopher Gibbs, Tobias P. Neville, Jason Millichamp, Paul R. Shearing, Daniel J. L. Brett, Journal Electrochemical Society, 2017, 164, F1551-F1555.
Abstract
Many electrochemical studies exist using the acidic ionomer Nafion as a binder in the ink formulation when operating in high pH systems. However, Nafion acts as an ionic insulator for OH−, and for reactions such as the hydrogen oxidation reaction, the transport of OH− to the catalyst surface is of utmost importance when elucidating the performance of a catalyst. This work demonstrates that when using an alkaline polymer binder in the ink, the apparent activity of a commercially synthesized Pt/C catalyst is increased due to a lower diffusion resistance for the reaction. In order to obtain accurate values for kinetic data in alkaline media, the use of the acidic binder should be avoided.
- Carbon nitrides: synthesis and characterization of a new class of functional materials
- Thomas Miller, Ana Belen Jorge, Theo Suter, Andrea Sella, Furio Cora, Paul McMillan, Phys. Chem.Chem.Phys., 2017, 19, 15613–15638.
Abstract
Carbon nitride compounds with high N : C ratios and graphitic to polymeric structures are beinginvestigated as potential next-generation materials for incorporation in devices for energy conversion and storage as well as for optoelectronic and catalysis applications. The materials are built from C- and N-containing heterocycles with heptazine or triazine rings linked via sp2-bonded N atoms (N(C)3 units) or –NH– groups. The electronic, chemical and optical functionalities are determined by the nature of the local to extended structures as well as the chemical composition of the materials. Because of their typically amorphous to nanocrystalline nature and variable composition, significant challenges remain to fully assess and calibrate the structure–functionality relationships among carbon nitride materials. It is also important to devise a useful and consistent approach to naming the different classes of carbon nitride compounds that accurately describes their chemical and structural characteristics related to their functional performance. Here we evaluate the current state of understanding to highlight key issues in these areas and point out new directions in their development as advanced technological materials. 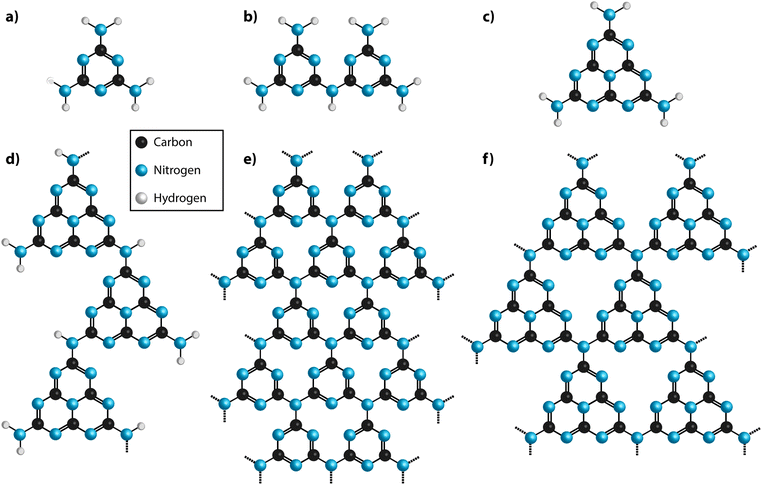
- Correlation between the proton conductivity and diffusion coefficient of sulfonic acid functionalized chitosan and Nafion composites via impedance spectroscopy measurements. I. Ressam, M. Lahcini, A. Belen Jorge, H Perrot, O. Sel, Ionics, 2017, 23, 2221-2227.
Abstract
Electrochemical Impedance Spectroscopy (EIS) was employed to estimate the global transverse proton diffusion coefficient, DH+, in sulfonic acid functionalized sustainable chitosan (CS-SO3H)/Nafion composite films. In contrast to conventional conductivity measurements, EIS measurements were performed at room temperature with a film/liquid interface. In this configuration, the measure of the bulk proton transport is correlated to the DH+ of the membranes which is close to 1.1 × 10−6 cm2 s−1 and 0.33 × 10−6 cm2 s−1 with and without CS-SO3H, respectively. These DH+ values permitted the proton conductivity (σH+) ratio (∼3.9) between the Nafion/CS-SO3H composite and pristine Nafion films to be estimated by using the Nernst-Einstein relationship. This ratio presents a good agreement with that obtained for the σH+ of bulk membranes (∼3.2) measured at 30 °C and 90% RH. The agreement between the σH+ ratios validates our methodology for DH+ estimation by EIS and suggests that the more than three times enhanced σ+H is governed by the ∼3 times higher DH+ in the presence of CS-SO3H. - Graphitic Carbon Nitride as a Catalyst Support in Fuel Cells and Electrolyzers,
- Noramalina Mansor, Thomas S Miller, Ishanka Dedigama, Ana Belen Jorge, Jingjing Jia, Veronika Brázdová, Cecilia Mattevi, Chris Gibbs, David Hodgson, Paul R Shearing, Christopher A Howard, Furio Corà, Milo Shaffer, Daniel JL Brett, Paul F McMillan, Electrochim. Acta, 2016, 222, 44-57.
Abstract
Electrochemical power sources, such as polymer electrolyte membrane fuel cells (PEMFCs), require the use of precious metal catalysts which are deposited as nanoparticles onto supports in order to minimize their mass loading and therefore cost. State-of-the-art/commercial supports are based on forms of carbon black. However, carbon supports present disadvantages including corrosion in the operating fuel cell environment and loss of catalyst activity. Here we review recent work examining the potential of different varieties of graphitic carbon nitride (gCN) as catalyst supports, highlighting their likely benefits, as well as the challenges associated with their implementation. The performance of gCN and hybrid gCN-carbon materials as PEMFC electrodes is discussed, as well as their potential for use in alkaline systems and water electrolyzers. We illustrate the discussion with examples taken from our own recent studies.